
Hasan K (2020) Methyl salicylate functionalized magnetic chitosan immobilized palladium nanoparticles: an efficient catalyst for the Suzuki and heck coupling reactions in water. Han HS, Jiang SN, Huang MY, Jiang YY (1996) Catalytic hydrogenation of aromatic nitro compounds by non-noble metal complexes of chitosan. Hajipour A, Boostani E, Mohammadsaleh F (2015) Proline-functionalized chitosan–palladium (ii) complex, a novel nanocatalyst for C–C bond formation in water. In: Mozafari M, Chauhan NPS (eds) Advanced functional polymers for biomedical applications. Hada D, Rathore K, Barupal T, Chundawat NS, Sharma K, Chauhan NPS (2019) Grafted biopolymers I: methodology and factors affecting grafting. Chem Soc Rev 40:5151–5169įuruhashi T, Beran A, Blazso M, Czegeny Z, Schwarzinger C, Steiner G (2009) Pyrolysis GC/MS and IR spectroscopy in chitin analysis of molluscan shells.

J Am Chem Soc 127:2485–2495įortman GC, Nolan SP (2011) N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: a perfect union. Chem Rev 106:2651–2710ĭorta R, Stevens ED, Scott NM, Costabile C, Cavallo L, Hoff CD et al (2005) Steric and electronic properties of N-heterocyclic carbenes (NHC): a detailed study on their interaction with Ni(CO) 4. Mater Today Chem 14:100184Ĭorbet J-P, Mignani G (2006) Selected patented cross-coupling reaction technologies. Int J Polym Mater 61:57–71Ĭhauhan N, Hosmane N, Mozafari M (2019) Boron-based polymers: opportunities and challenges. J Appl Polym Sci 122:573–585Ĭhauhan NP, Kataria P, Chaudhary J, Ameta SC (2012) Synthesis, characterization, and thermal studies of terpolymers derived from vanillin, furfural, and halo-substituted acetophenones. Walter de Gruyter GmbH & Co KG, BerlinĬhauhan NP, Ameta R, Ameta SC (2011) Synthesis, characterization, and thermal degradation of p-chloroacetophenone oxime based polymers having biological activities. J Ind Eng Chem 19:1014–1023Ĭhauhan NPS, Chundawat NS (2019) Inorganic and organometallic polymers. J Appl Polym Sci 83:2188–2194Ĭhauhan NPS (2013) Structural and thermal characterization of macro-branched functional terpolymer containing 8-hydroxyquinoline moieties with enhancing biocidal properties. Aust J Chem 55:73–78Ĭhang Y, Wang Y, Su Z (2002) Preparation of chitosan-bound nitrobenzaldehyde metal complexes and studies on its catalytic oxidative activity and reactive mechanism. Acc Chem Res 41:1439īuisson P, Quignard F (2002) Polysaccharides: natural polymeric supports for aqueous phase catalysts in the allylic substitution reaction. Green Chem 17:1243–1248īarder TE, Walker SD, Martinelli JR, Buchwald SL (2005) Catalysts for Suzuki–Miyaura coupling processes: scope and studies of the effect of ligand structure. Environ Health Perspect 114:A 84-Aīaig RN, Vaddula BR, Nadagouda MN, Varma RS (2015) The copper–nicotinamide complex: sustainable applications in coupling and cycloaddition reactions. Macromol Symp 80:257–263Īnderson EL (2006) Phosphine toxicity: ethical questions. RSC Adv 5:27533–27539Īn Y, Yuan D, Huang MY, Jiang YY (1994) Selective hydrogenation of chloronitrobenzenes catalyzed by palladium complex of silica-supported chitosan. This study furnishes an economic and eco-friendly catalyst for organic transformation in sustainable chemistry.Īffrose A, Suresh P, Azath IA, Pitchumani K (2015) Palladium nanoparticles embedded on thiourea-modified chitosan: a green and sustainable heterogeneous catalyst for the Suzuki reaction in water. CPIP complex has also been found to give magnificent results in Suzuki–Miyaura and Heck cross-coupling reactions, and therefore, using this green catalyst, the toxic phosphine ligand can be excluded from cross-coupling reactions. This catalyst shows an excellent activity in the reduction of toxic pollutant nitrobenzene to less toxic aniline.

TG-DSC study suggested that the catalyst is thermally stable up to 300 ☌.
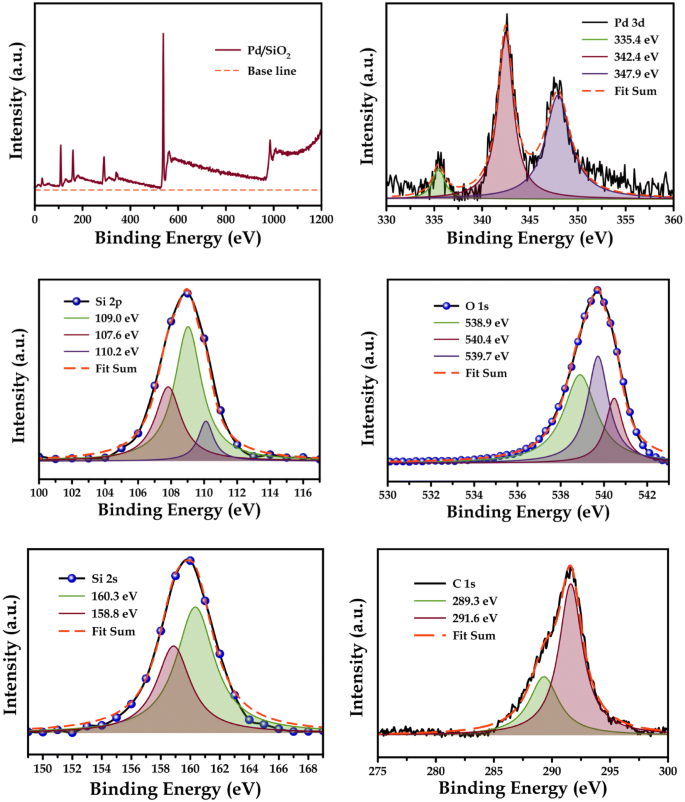
The formed CPIP has been extensively characterized with respect to raw chitosan utilizing methods including FT-IR, pyrolysis GC–MS, XRD, XPS, FE-SEM, EDS, TGA-DTG and DSC. In this work, the modification of chitosan using 2-acetyl pyridine has been used to prepare an intermediate, chitosan pyridyl imine (CPI), in first step and then in second step it is further reacted with Pd(OAc) 2 to develop chitosan pyridyl imine palladium (CPIP) complex catalyst in a very simplistic way.


 0 kommentar(er)
0 kommentar(er)
